Specific Aim: When a long skeletal muscle cell is subjected to an applied field, the transmembrane potential distribution becomes spatially non-uniform because of the non-homogeneous distribution of membrane pores. In order to use the proposed model to study pore population under such conditions, it is necessary to know the steady-state transmembrane potential distribution under an applied field. The 3-D distribution of transmembrane potential of a muscle cell will be imaged using optical sectioning confocal microscopy and a voltage-sensitive fluorescent dye. The 3-D image of the cell will be reconstructed from a series of optical sections of the cell. A blind deconvolution method will be developed to deblur the optical sections prior to image reconstruction. The deblurred sections will be used to visualize the 3-D distribution of transmembrane potential.
Experimental Design and Methods: A theoretical model of electroporation will be used to estimate membrane pore distribution corresponding to the measured transmembrane potential distribution. Thus, the values of model parameters should reflect experimental measurements. This is ensured by using experimentally measured transmembrane potential distribution to evaluate parameters relating to steady-state distribution of membrane pores. Experimental measurement of transmembrane potential will be based on optical measurement of spatial distribution of a voltage-sensitive dye bound to the membrane of a skeletal muscle cell.
Potentiometric Dye: Several voltage-sensitive indicator dyes have been developed to measure relative changes in transmembrane potential induced by an external stimulus. These dyes permit the mapping of membrane potential along a cell surface with a resolution limited only by microscope optics. They also have a rapid response that makes it possible to follow the fastest conceivable changes in transmembrane potential (Loew, 1992). Di-8-ANEPPS, a p-aminostyryl-based probe which works by an electrochromic mechanism will be used in the measurement of transmembrane potential. This dye was selected because of its slow internalization, high signal-to-noise ratio and large potentiometric fluorescence change. Di-8-ANEPPS (mol.wt. 592.88) internalizes less than 40% into the cytoplasm during the hour following initial staining; di-8-ANEPPS labeling results in SNRs as high as 40:1; average fluorescence changes of 15% per 100 mV could be realized using this dye (Rohr and Salzberg, 1994).
Skeletal Muscle Cell: Rat skeletal muscle cells from flexor digitorum brevis muscle will be used in the measurement of transmembrane potential. The reason for using this cell type lies in its shape and dimensions. The effective field strength across a cell membrane can be many orders of magnitude higher than the external applied field strength. In a uniform extracellular electric field, cell membrane experiences an induced voltage drop that scales with cell length. Therefore, longer cells are more susceptible to damage than smaller cells (Gaylor et al. 1988). Large voltage drops induced on membranes of long cells by an external field is also of importance in electroporation applications. This fact indicates that temperature rise effects encountered in electroporation can be contained by using smaller pulses on physically long cells. Thus, study of membrane-field interaction will focus on physically and electrically long cells, such as skeletal muscle cells.
Staining Protocol: Stock solutions (5 mg/ml) of di-8-ANEPPS (Molecular Probes, Eugene, OR) will be prepared by dissolving in a 1:1 mixture of ethanol and 75% w/w DMSO and 25% w/w Pluronic F127 (BASF, Wyandotte, MI). The use of DMSO and F127 will help in ensuring that the dye comes into contact and anchors into the cell membrane. The stock solutions will be tightly capped and stored in a freezer. Fresh staining solutions will be prepared immediately prior to experiments by adding appropriate amounts of gently warmed stock solution to phosphate buffered saline solution (PBS) at pH=7.4. The concentration of the stock solvent in the final staining solution will range from 0.5-1%. Skeletal muscle cells will be stained at room temperature for 15-20 minutes.

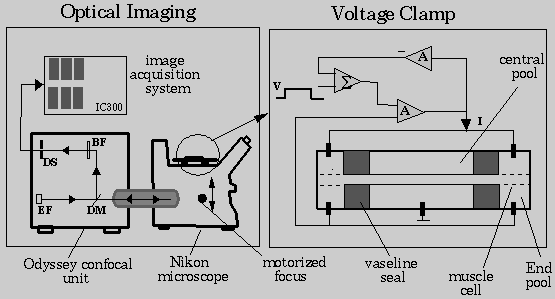
Confocal Microscope: An imaging system built around Odyssey
(Noran Instruments, Inc.), a laser scanning slit confocal system, will be used
in the measurement of transmembrane potential (Fig. 8). The confocal system will be
used as an attachment to an inverted Nikon Diaphot microscope. The Odyssey
system was selected because it facilitates
high-resolution, real-time imaging at multiple excitation wavelengths.
It uses a triple dichroic mirror to generate excitation beams at 457, 488 and
529 nm wavelengths. This feature is useful in ratiometric imaging that is
often employed to counter the problem of non-uniformity in dye concentration
encountered in potentiometric measurements. Real-time laser scanning
is achieved using a slit detector in place of a pin-hole detector used in
conventional confocal systems. Though this constrains confocality to two
dimensions, the performance meets the requirements of the proposed measurement
method. Real-time scanning of the specimen is useful in recording rapid
changes
in transmembrane potential, limited only by the signal-to-noise ratio offered
by the potentiometric dye. An optimal trade-off between image resolution
and sensitivity can be obtained with Odyssey by using software selectable
detector slits of 10, 15, 25, 50 and 100  m widths. The fluorescence
output of the confocal microscope is fed through a RS170 interface to an
image processing system.
m widths. The fluorescence
output of the confocal microscope is fed through a RS170 interface to an
image processing system.
Imaging System: An IC300 image processing system (Inovision Corp., Raleigh, NC) will be used to acquire and process fluorescence video output from the confocal microscope. This system contains an 8-bit video board that digitizes 512x480 images at 30 frames per second. This is augmented by six 512x512x8-bit frame buffers and two ALU's which enable video rate arithmetic and logical operations using 16-bit data paths. This feature will be used in real-time background subtraction and feature extraction that will be required in transmembrane potential imaging. The function of the Odyssey system will be controlled from a Sun IPX workstation, the front-end of IC300, using Isee (Inovision Corp., Raleigh, NC), an icon map based image acquisition and processing software.
Fluorescence Experiment Protocol: One- to three-mm
long adult skeletal muscle cells will be isolated by enzymatic digestion
from rat flexor digitorum brevis (FDB) and maintained in culture medium.
The Dulbecco's Modified Eagle
Medium (DMEM) in which cells grow will be washed out prior to staining
with 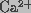 -free phosphate buffered saline solution (pH 7.4). Cells
will be stained using the protocol described earlier. Following staining, the
residual dye will be washed out with
-free phosphate buffered saline solution (pH 7.4). Cells
will be stained using the protocol described earlier. Following staining, the
residual dye will be washed out with 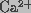 . Cells will then be placed
on the microscope stage and imaged using a 40x, 1.4 N.A. objective.
Fluorescence measurements will be performed at excitation/emission wavelengths
of 488/515 nm. To improve the signal-to-noise ratio, 64 successive frames will
be averaged to generate each image. A complete focus-through series of 60
images will be acquired at a focal spacing of 1
. Cells will then be placed
on the microscope stage and imaged using a 40x, 1.4 N.A. objective.
Fluorescence measurements will be performed at excitation/emission wavelengths
of 488/515 nm. To improve the signal-to-noise ratio, 64 successive frames will
be averaged to generate each image. A complete focus-through series of 60
images will be acquired at a focal spacing of 1  m.
m.
Reconstruction of Optical Sections: The nearest neighbor
deconvolution method used thus far accounts for scatter contributions from
only adjacent focal planes. This approach is justified for reconstructing
images obtained using narrow slit detectors. However, as the measured
PSFs show, there is a considerable amount of scatter from planes farther
than adjacent focal planes when slits of width greater than 25  m are
used.
m are
used.
The PSF of an optical microscopy system depends on a number of parameters such as the refractive indices of the components in the light path, excitation wavelength, coverslip thickness and depth of point source (McNally et al. 1994). This entails the measurement of PSF under many possible experimental conditions. On the other hand, empirically derived PSF estimates do not account for all the factors that introduce blurring in such a system. Thus, a blind iterative deconvolution (BID) scheme will be used to restore fluorescence microscopy images. Using this technique, both the restored image and the degrading PSF will be recovered from a z-series of the muscle cell.
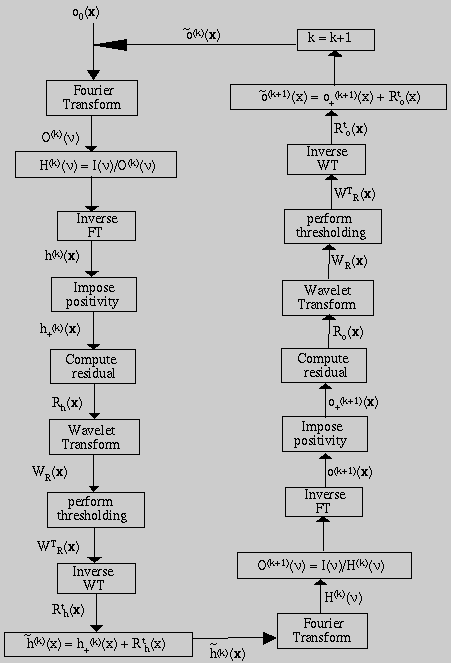
The general blind iterative deconvolution approach proposed by Ayers and Dainty (1988) will be implemented with a modified wavelet based restoration step. The basic approach, outlined in Fig. 9, is to alternately deconvolve the blurred image with the PSF and then with the restored image. A set of physical constraints are applied after each iteration.
The observed image is modeled as the output of a three-dimensional finite impulse response linear shift-invariant system. The blurred image i(x) can be described as
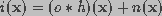 (15)
(15)
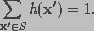 (16)
(16)
In Fourier space, we have
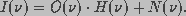 (17)
(17)
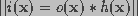 .
If the noise is modeled as a Gaussian process, then an iterative
approach for computing maximum likelihood estimates may be used. The Van
Cittert restoration method (Starck and Bijaoui, 1994) uses such an iterative
scheme.
.
If the noise is modeled as a Gaussian process, then an iterative
approach for computing maximum likelihood estimates may be used. The Van
Cittert restoration method (Starck and Bijaoui, 1994) uses such an iterative
scheme.
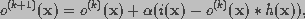 (18)
(18)
 is a convergence parameter. In this equation, the object
distribution is modified by adding a term proportional to the residual.
The residual at iteration k is defined as
is a convergence parameter. In this equation, the object
distribution is modified by adding a term proportional to the residual.
The residual at iteration k is defined as
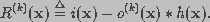 (19)
(19)
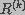 can be expressed as the sum of
its p wavelet planes and the final smooth plane
can be expressed as the sum of
its p wavelet planes and the final smooth plane
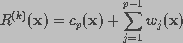 (20)
(20)
The wavelet coefficients provide a mechanism to extract only the significant structures from the residuals at each iteration. Normally, a large part of these residuals are statistically non-significant. The significant residual is expressed as
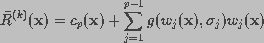 (21)
(21)
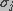 is the standard deviation of the noise at scale j, and
g is a function which is defined by:
is the standard deviation of the noise at scale j, and
g is a function which is defined by:
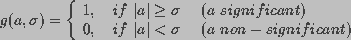 (22)
(22)
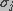 is estimated from the standard
deviation of the noise in the blurred image.
is estimated from the standard
deviation of the noise in the blurred image. The individual optical sections will be restored using the modified blind deconvolution procedure. The 3-D potentiometric dye distribution will be visualized using the restored optical sections. Reconstructed fluorescence image of skeletal muscle cells under a number of small transmembrane potentials will be used in determining the steady-state 3-D transmembrane potential distribution.
Preliminary Studies: A theoretical model of electroporation will be used to estimate membrane pore distribution corresponding to a measured transmembrane potential distribution. Thus, the values of model parameters should reflect experimental measurements. This is ensured by using experimentally measured transmembrane potential distribution to evaluate parameters relating to steady-state distribution of membrane pores.
Experimental measurement of transmembrane potential will be based on optical measurement of spatial distribution of a voltage-sensitive dye bound to the membrane of a skeletal muscle cell. The preliminary work on the experimental measurement of transmembrane potential was devoted to the design, construction and calibration of a transmembrane potential measurement system. An experimental protocol for imaging potentiometrically-stained skeletal muscle cells was also developed. A procedure for 3-D reconstruction of skeletal muscle cells stained with a voltage-sensitive dye was designed and tested.
Experimental Measurement of PSF: An optical imaging system will be used in measuring transmembrane potential distribution to evaluate the parameters of the the electroporation model. One of the primary prerequisites in such measurements is the characterization of the optics used in imaging the biological system of interest. The three-dimensional image-forming properties of the Odyssey confocal microscope that will be employed in these measurements were experimentally measured (Gowrishankar et al. 1993). The 3-D optical transfer function (OTF) computed from these measurements will be used in reconstructing 3-D images of muscle cells from their optical sections.
The experimental design criterion in laser scanning confocal microscopy measurements is to use low laser power with a narrow slit to generate high-resolution images. Point spread function (PSF) measurements presented above represent the tradeoff between the sensitivity and resolution of the confocal system. This will be used in arriving at an optimal choice of confocal slit and excitation power level for transmembrane potential measurements.

 m diameter. The optical
sections (0.6
m diameter. The optical
sections (0.6  m
apart) were obtained using a 40x, 1.4 N.A. lens with a 488 nm excitation
source. An emission filter 515 nm was used. Each image is a temporal
average of 64 frames. Image degradation away from the focal plane is
more prominent for a 100
m
apart) were obtained using a 40x, 1.4 N.A. lens with a 488 nm excitation
source. An emission filter 515 nm was used. Each image is a temporal
average of 64 frames. Image degradation away from the focal plane is
more prominent for a 100  m slit (top) than for a 25
m slit (top) than for a 25
 m slit (bottom).
m slit (bottom).
The PSF of the confocal microscope was measured from
images of fluorescently labeled microspheres. A series of images was acquired
by stepping the focus motor at 0.2  m spacing through the
beads.
Fig. 6 shows a series of images of the fluorescent beads acquired
at different amounts of defocus for 100
m spacing through the
beads.
Fig. 6 shows a series of images of the fluorescent beads acquired
at different amounts of defocus for 100 m and 25
m and 25  m slit widths.
The decrease in sensitivity and an improved resolution with a 25
m slit widths.
The decrease in sensitivity and an improved resolution with a 25  m
slit compared to 100
m
slit compared to 100  m is evident from the images.
Degradation of high frequency information in a direction
parallel to the slit was evident in the OTF of a larger width slit.
This corresponds to the missing cone phenomenon observed in conventional
bright-field microscopes and agrees with the theoretical prediction that
properties of a confocal microscope with wide slits approach that of a
conventional microscope.
m is evident from the images.
Degradation of high frequency information in a direction
parallel to the slit was evident in the OTF of a larger width slit.
This corresponds to the missing cone phenomenon observed in conventional
bright-field microscopes and agrees with the theoretical prediction that
properties of a confocal microscope with wide slits approach that of a
conventional microscope.
Fluorescence imaging of potentiometrically-stained skeletal muscle cells
inherently have a low signal-to-noise ratio. This is due to the poor
quantum yield
of these dyes. In addition, the use of a narrow slit detector results in the
detection of only a small fraction of fluorescent light emitted from the
specimen. Use of laser excitation at high power levels to offset the low
signal strength often results in photobleaching and related phototoxic
effects. Thus, an optimal tradeoff between sensitivity and resolution is
required for imaging transmembrane potential distribution. Based on the point
spread function measurements, a 100  m slit width was selected for
imaging skeletal muscle cells stained with a voltage-sensitive dye.
m slit width was selected for
imaging skeletal muscle cells stained with a voltage-sensitive dye.
Three-dimensional Reconstruction of Confocal Images: One of the assumptions of the proposed model was that the electrical properties of the cylindrical cell membrane are uniform around the long axis. The validity of this assumption will be verified by measuring the three-dimensional distribution of transmembrane potential under an imposed electric field. Three-dimensional reconstruction of fluorescently-stained skeletal muscle cells is an intermediate step in the measurement of transmembrane potential distribution.

 m focal spacing were acquired for
reconstructing the cell.
A nearest neighbor deconvolution procedure was used in the reconstruction.
The optical sections were corrected for background and shade variations
prior to reconstruction.
m focal spacing were acquired for
reconstructing the cell.
A nearest neighbor deconvolution procedure was used in the reconstruction.
The optical sections were corrected for background and shade variations
prior to reconstruction.
In order to generate a three-dimensional reconstruction of a fluorescent
image of a skeletal muscle cell, a number of optical sections of the cell
were acquired by stepping the fine focus of the microscope in increments of 1
 m. Figure 7 (left) shows a series of optical sections
of a di-8-ANEPPS-stained skeletal muscle cell. These images were corrected
for background variations by subtracting them from a background image acquired
prior to the collection of the series. Images were also corrected for
intensity variations within the image plane by modeling the source profile
as a Gaussian distribution.
m. Figure 7 (left) shows a series of optical sections
of a di-8-ANEPPS-stained skeletal muscle cell. These images were corrected
for background variations by subtracting them from a background image acquired
prior to the collection of the series. Images were also corrected for
intensity variations within the image plane by modeling the source profile
as a Gaussian distribution.
These optical sections were used to reconstruct the three-dimensional image
of the skeletal muscle cell by nearest-neighbor deconvolution with a
non-negative constraint (Agard et al. 1989;
Gruenbaum et al. 1984). The
simplest scheme for deblurring optical section data sets uses image data from
a single focal plane above and a single focal plane below to correct the
central focal plane. This method provides satisfactory results for images
obtained using a confocal microscope because of the excellent scatter-rejection
capabilities of the microscope. Figure 7 (right) shows a reconstructed
skeletal muscle cell computed from optical sections of Fig. 7 (left). Because
of the sensitivity constraints imposed by the measurement process, it was
determined that a slit of 100  m is required for imaging
potentiometrically-stained skeletal muscle cells. Thus, it might be necessary
to deblur contributions from more distant image planes.
m is required for imaging
potentiometrically-stained skeletal muscle cells. Thus, it might be necessary
to deblur contributions from more distant image planes.
Results shown in Fig. 7 demonstrate the capability to measure transmembrane potential distribution using a potentiometric dye and three-dimensional reconstruction from optical sections. Fluorescence microscopy methods will also be used in conjunction with the voltage clamp arrangement to determine the extent of spatial non-uniformity in transmembrane potential distribution introduced by electroporation.