Background
The discovery (of bioelectromagnetism) was a great stimulus for biological
experiments. They soon became the favourite entertainment in the scientific
salons of the aristocracy of the Enlightenment. One of the leading
experimenters of the time was the Abbé Jean-Antoine Nollet. As much showman
as physicist, Nollet had 180 guardsmen at Versailles to join hands in a row,
while the last two men touched a Leyden jar. This made them all jump up
simultaneously, much to the amusement of the king.
-- Bioelectrodynamics and Biocommunication. Eds. M-W. Ho, F-A. Popp and U. Warnke.
World Scientific: New Jersey. 1994.
A common consequence of cell membrane interaction with an electric field
is a significant change in transmembrane potential, conductivity and
permeability.
These changes result from the formation of aqueous pores on the cell membrane.
This is the underlying mechanism in such events as electroporation, nerve
conduction and high-voltage electric injury. The model to be developed in
this project will be useful in estimating the dynamics of these pores in
such events. This section discusses the biological events involving
electroporation, features of existing theoretical models and experimental
techniques.
Electric Field Induced Changes in Membrane Properties
Changes in transmembrane potential and membrane conductance accompany many
instances of membrane-field interaction. These events include both useful
and harmful effects of an electric field on biological systems. Examples
of such phenomena include electroporation, nerve conduction and high-voltage
injury.
Electroporation: An important application of
strong electric fields on cells is in membrane electroporation.
Electroporation of cells uses intense electric pulses to alter membrane
permeability for increased uptake of drugs, molecular probes, and DNA.
Electroporation has many applications in medicine and biology, such as
introduction of DNA into living cells, fusion of cells, insertion of proteins
into cell membranes, improving drug delivery and its effectiveness in
chemotherapy of cancerous cells, and alteration of genetic expression in living
cells (Tsong, 1991;
Neumann et al. 1989;
Chang et al. 1992;
Blank, 1993;
Weaver, 1993;
Orlowski and Mir, 1993).
Electroporation and its related phenomena such
as electrofusion and bleb formation reflect the basic bioelectrochemistry of
cell membranes and are thus important in the study of membrane structure and
function.
The underlying mechanism of electroporation involves increasing membrane
permeability using a strong electric field. Under normal physiological
conditions, a bilayer lipid membrane made of lipid
extracts of cells is a good barrier for ions and hydrophilic molecules. The
membrane conductance to 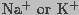 is typically
is typically 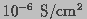 or
smaller (Tien, 1974). This permeation barrier is readily modified by imposing
a transmembrane potential. When an intense transmembrane electric
field exceeding the dielectric strength of a cell membrane is applied, the
membrane specific conductance increases dramatically and can reach as high
as
or
smaller (Tien, 1974). This permeation barrier is readily modified by imposing
a transmembrane potential. When an intense transmembrane electric
field exceeding the dielectric strength of a cell membrane is applied, the
membrane specific conductance increases dramatically and can reach as high
as 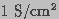 in microseconds (Hibino et al. 1993). Cell membranes can
sustain as much as 1 V of transmembrane potential, i.e., an electric field
strength of 2,000 kV/cm, when microsecond to millisecond electric pulses are
used (Coster et al. 1975).
in microseconds (Hibino et al. 1993). Cell membranes can
sustain as much as 1 V of transmembrane potential, i.e., an electric field
strength of 2,000 kV/cm, when microsecond to millisecond electric pulses are
used (Coster et al. 1975).
The proposed model can be used to determine pore distribution and membrane
conductance for pulses used in electroporation. These
parameters will be useful in selecting pulse parameters in electroporation
applications based on the desired size of pores. The strong field applied
during electroporation causes local heating which could also be estimated using
the predictions of the proposed model.
High-voltage Electric Shock: High-voltage electrical shock
is another event in which a strong electric field leads to membrane
poration and consequent changes in membrane electrical properties. Such
intense fields elevate the transmembrane potential by hundreds of mV thus
decreasing the energy barrier allowing the creation of large radii pores.
Knowledge of electropore population is necessary for the design of
a therapeutic treatment for electrical trauma.
High-voltage electrical trauma frequently leads to selective, yet, extensive
destruction of muscle and nerve tissue (Lee, 1991). Many of the immediate
clinical signs of a major electrical injury relate to neuromuscular damage
revealed by intense muscular spasm. These observations suggest that muscle cell
membrane rupture is one of the primary mechanisms of tissue damage in
high-voltage shock. Theoretical and experimental evidence suggests that long
skeletal muscle and nerve cells are vulnerable to plasma membrane rupture by
electrical breakdown (Gaylor et al. 1988). This breakdown occurs as a result
of the formation of pores on cell membrane leading to an increased membrane
conductivity and diffusive permeability and to changes in the spatial
distribution of transmembrane potential. Therefore, the
change in transmembrane potential distribution is a measure of the
physiologic state of a cell following an electric shock. Investigation of
changes in membrane properties introduced by an electric field is crucial
for understanding the consequences of high-voltage electric shock and in
designing an effective treatment for these injuries.
One method of treating electrical injury is by inducing the sealing of
membrane pores. Nonionic block copolymer surfactants have been shown to seal
electropermeabilized skeletal muscle cells (Lee et al. 1992). These surfactants
form a family of non-toxic, non-ionic surface-active agents containing a
hydrophobic propylene oxide chain sandwiched between two sequences of
hydrophilic ethylene oxide chains. The properties of these surfactants are
dependent on the ratio of the length of the hydrophobic chain to that of the
hydrophilic chain. The choice of surfactants for sealing of membrane pores
depends on the size and population of such pores. Extension of the proposed
model to breakdown potentials will be useful in designing optimal surfactants
for treating electrical injury.
The proposed non-uniform cable model will be designed to study electropore
distribution in terms of electrical properties of the cell membrane. Changes
in these properties dictate the kinetics of pore formation and evolution. The
linear cable model will be used in the proposed work to represent the electrical
properties of a skeletal cell membrane.
Linear Cable Theory of Electrophysiologic Behavior
Membrane pores arising from the application of an external electric field
in such events as electroporation and high-voltage electric injury contribute
to changes in the electrical properties of the cell membrane. The objective
of this project is to estimate these changes in membrane electrical
properties and to determine the distribution of membrane pores.
Existing models depict a cell membrane as having spatially uniform electrical
properties (Jack et al. 1975). According to these models, the cell membrane
acts as an insulating shell in a continuous conductor causing the voltage
across the cell diameter to appear mainly across the relatively thin membrane.
This results in a field amplification of a few orders of magnitude. This field
amplification at the cell membrane level is a trigger for the initiation of
many cell biological effects. Therefore, it is critical to be able to
determine the perturbations to the transmembrane potential induced by
exposure to an electric field if a full understanding of the ensuing
cellular responses is to be reached.
Conduction of the cell membrane has been studied extensively with the
standard cable model where a cylindrical fiber is considered to behave like a
co-axial cable with leakage between the intracellular and extracellular media
(Rall, 1977;
Joyner et al. 1983;
Katz, 1966). In a cable model,
electrical properties of the plasma membrane are represented by a series of
parallel resistors and capacitors. This lumped parameter circuit model of
the membrane is combined with the resistances of the intracellular and
extracellular media to analyze the effects of an external field. The cable
equations that represent the cable circuit are solved to determine the spatial
distribution of induced transmembrane potential
(Ranck, 1963;
Sten-Knudsen, 1960).
The cable circuit model forms the basis for representing transmembrane potential
distribution in the proposed model with a modification that the cell membrane
is assumed to be spatially non-uniform. The non-uniformity results from the
presence of membrane pores which is modeled by the membrane pore theory.
Membrane Pore Theory
Lack of a rigorous experimental method to measure membrane pore dynamics
has prompted the development of several models to study the formation of
membrane pores in the context of electroporation
(Abidor et al. 1979;
Sugar and Neumann, 1984;
Weaver and Mintzer, 1986;
Saulis and Venslauskas, 1993).
Although the mechanism by which electroporation occurs is not completely
understood, it is generally believed that a rapid structural rearrangement
of membrane occurs, whereby many aqueous pathways ("pores") perforate the
membrane (Freeman et al. 1994).
Early attempts to use the presence of local defects (pores) to
explain increased membrane permeabilization were made by
Abidor et al. (1979) .
Sugar and Neumann (1984) treated electroporation as a stochastic
process and modeled the aqueous pores as periodic block structures.
Independently,
Weaver and Mintzer (1986) developed a model based on the
hypothesis that a large number of transient aqueous pores continually present
on the bilayer membrane expand, leading to increased conduction. This model
was extended to study pore density and molecular transport under
charge-injection conditions
(Barnett and Weaver, 1991;
Freeman et al. 1994).
Recently, (Saulis and Venslauskas 1993) developed a model assuming that
the creation and disappearance of metastable pores are random one-step
processes.
The aqueous pore theory (Weaver and Mintzer, 1986) has been refined to
estimate pore distribution and molecular transport across bilayer lipid
membranes
(Barnett and Weaver, 1991;
Freeman et al. 1994). This model will be
used in the proposed work as the basis to represent the creation and
evolution of membrane pores. According to the membrane pore theory, transient
pores appear on the membrane as thermal defects. These pores are assumed to
spontaneously contract in the absence of expansive forces. Random
bombardment of water molecules generates a fluctuating macroscopic pressure,
leading to a distribution of pore radii. An applied field contributes to this
force leading to the expansion of the existing pores to larger radii. The
distribution of pores is thus described as a balance between the restoring
force and the field induced expanding force (Barnett and Weaver, 1991).
The frameworks of aqueous pore theory and cable theory are used in this
project to model the changes in the electrical properties of a skeletal
muscle cell in the presence of an external field. These changes are coupled
to the pore energy to determine the population of membrane pores.
Experimental Techniques For Electroporation Studies
The kinetics of electroporation involve initial molecular reorganization of
the cell membrane and its secondary effect, pore formation, processes that
occur in nanoseconds to subsecond time scales (Tsong, 1991). This investigation
was motivated by the lack of experimental techniques capable of measuring
the kinetics of membrane property changes with adequate temporal resolution.
Experimental techniques used to study electroporation on skeletal muscle
cell membranes include morphological investigation using an electron
microscope with freeze fracture
(Chang and Reese, 1990) and permeability
studies employing a fluorescent dye
(Lee et al. 1992).
Tsong and colleagues (1991)
measured the conductivity of an erythrocyte suspension to compare membrane
electroporation and field-induced effects on 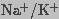 ATPase
(Teissie and Tsong, 1980;
Serpersu and Tsong, 1983). These methods involve
measurements taken after the electrical shock had occurred and have a time
resolution of 0.1 sec or greater.
ATPase
(Teissie and Tsong, 1980;
Serpersu and Tsong, 1983). These methods involve
measurements taken after the electrical shock had occurred and have a time
resolution of 0.1 sec or greater.
An alternate method permitting higher time resolution employs fast
potentiometric dyes to monitor transmembrane potential change.
Hibino et al. (1991)
used a submicrosecond imaging system to study electroporation in a sea urchin
egg. The charge injection/relaxation method has also been used in measuring
nanoampere magnitude transmembrane currents at high time resolution
(Benz and Zimmerman, 1980, 1981;
Chernomordik et al. 1983).
These methods are
useful only for the measurement of events occurring immediately after
an electric shock and not those occurring during the pulse.
Measurements of transmembrane current and potential in this project will
employ a voltage clamp technique. This method provides the capability
to measure transmembrane current during a constant transmembrane potential pulse. The
voltage clamp methodology has been used to study electroporation primarily
in artificial
lipid bilayer preparations
(Abidor et al. 1979;
Chernomordik et al. 1987;
Glaser et al. 1988) and
sparingly in live cells (O'Neill and Tung, 1991).
This investigation will use an improved double vaseline gap voltage clamp
(Chen and Lee, 1994) to measure transmembrane current and potential changes.
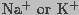 is typically
is typically 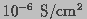 or
smaller (Tien, 1974). This permeation barrier is readily modified by imposing
a transmembrane potential. When an intense transmembrane electric
field exceeding the dielectric strength of a cell membrane is applied, the
membrane specific conductance increases dramatically and can reach as high
as
or
smaller (Tien, 1974). This permeation barrier is readily modified by imposing
a transmembrane potential. When an intense transmembrane electric
field exceeding the dielectric strength of a cell membrane is applied, the
membrane specific conductance increases dramatically and can reach as high
as 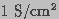 in microseconds (Hibino et al. 1993). Cell membranes can
sustain as much as 1 V of transmembrane potential, i.e., an electric field
strength of 2,000 kV/cm, when microsecond to millisecond electric pulses are
used (Coster et al. 1975).
in microseconds (Hibino et al. 1993). Cell membranes can
sustain as much as 1 V of transmembrane potential, i.e., an electric field
strength of 2,000 kV/cm, when microsecond to millisecond electric pulses are
used (Coster et al. 1975). 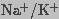 ATPase
(
ATPase
(