Broadly neutralizing Antibodies (bNAbs)
![]() Due to its high rate of mutation rate HIV evades antibodies and challenges vaccination protocols.
Due to its high rate of mutation rate HIV evades antibodies and challenges vaccination protocols.
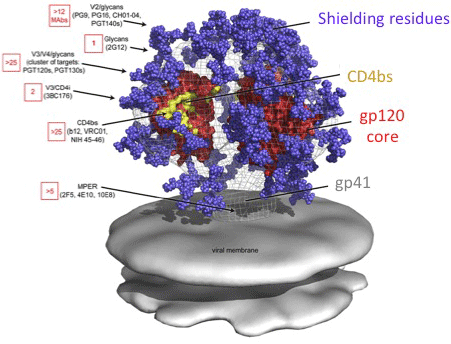
The HIV spike, the target of most antibodies, is shielded by easily mutable (low fitness cost) residues.
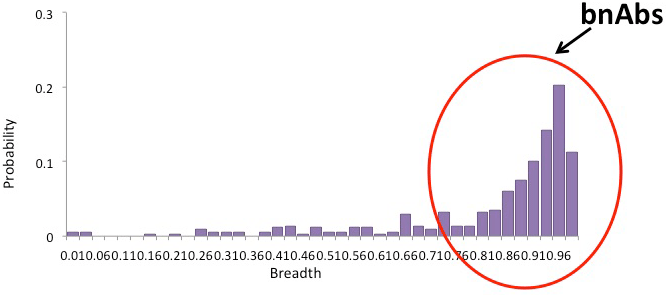
![]() Rare individuals, after several years and in low amounts, produce broadly neutralizing Antibodies (bNAbs),
Rare individuals, after several years and in low amounts, produce broadly neutralizing Antibodies (bNAbs),
capable of inactivating a broad range of viral mutants by targeting the conserved (CD4 binding) region.
![]() Can we devise a strategy to guide affinity maturation to create bNAbs?
Can we devise a strategy to guide affinity maturation to create bNAbs?
A potential strategy is to vaccinate with multiple mutant spikes sharing the same conserved elements.
Our computational model of affinity maturation suggests that a cocktail of such variants yields to low yields, with little breath:
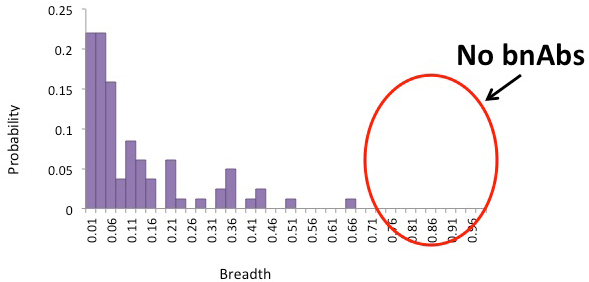
Simultaneous presence of variant antigens leads to conflicting selection forces that frustrate affinity maturation
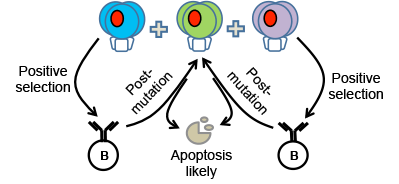
Simulations suggests successful outcome if the antigens are administered in sequence
Sequential immunization can focus B cell interactions on the conserved residues
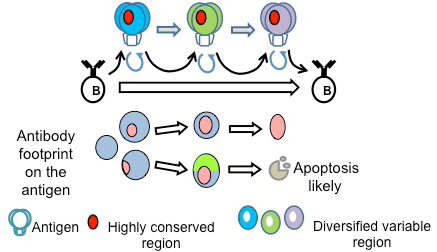
This prediction was successfully tested in mice (with Wittrup & Burton labs) [Wang et al, Cell 160, 785 (2015)] (off-line)
Essence of the challenge is to avoid termination of affinity maturation as well as a specialized outcome
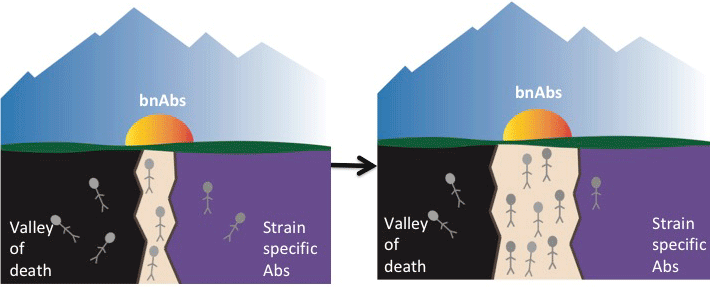
![]() A general strategy to elicit (control) bnAbs must:
A general strategy to elicit (control) bnAbs must:
Avoid frustration (leading to extinction of GC) [J.S. Shaffer, P.L. Moore, MK & AKC, PNAS 113, E7039 (2016)] (off-line)
Avoid rapid specialization to mutable epitopes
Boost B-cell lineages that can mutate towards conserved epitopes