Concentration Gradients
And Their Relation to Biased Random Walks
What is a concentration gradient?
Concentration of a chemical in a solution refers to how many of the chemical's molecules are sitting in a small volume of the solution. Concentration could be measured in molecules per liter, although molecules are so small compared to a liter that we usually use different units (just like we wouldn't want to measure the distance between the earth and the sun in inches). A gradient is a measurement of how much something changes as you move from one region to another. So a concentration gradient is a measurement of how the concentration of something changes from one place to another.
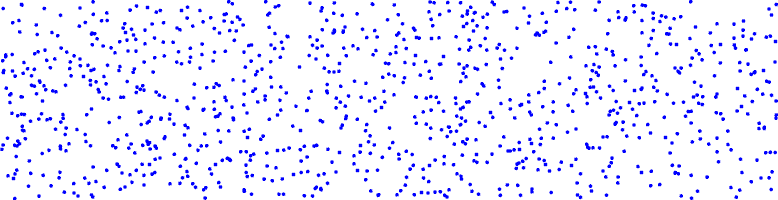
Let's give a few examples. If we picture each individual molecule as a little blue dot, a constant concentration of molecules (no gradient) would look like the picture below:

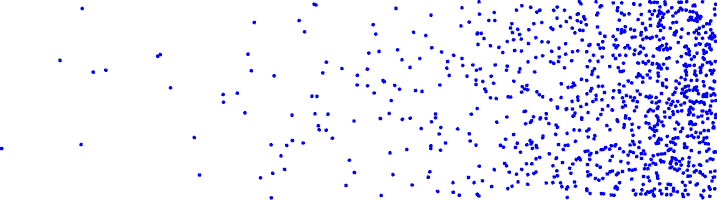
A concentration gradient, with a higher concentration of molecules on the right than on the left would look like the picture below:
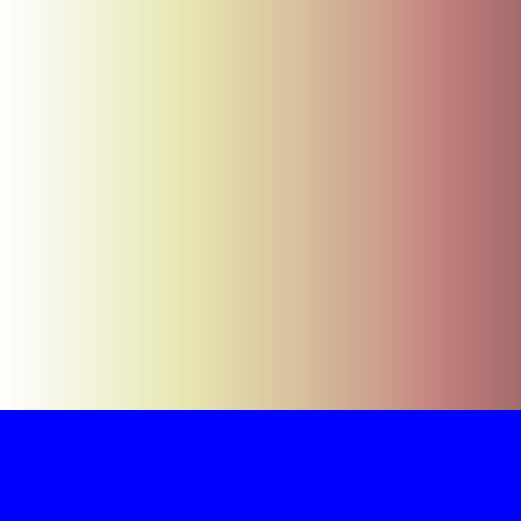
Now suppose we zoom out so that instead of seeing discrete little particles, we see a continuous gradient going from left to right (because molecules are so small, you can't see the individual molecules that make up food coloring in water, but you can see the color going from lighter to darker in some regions). If we represent high concentrations with dark pink and lower concentrations with white, a continuous concentration gradient would look like the picture below:
What does a concentration gradient have to do with a random walk?
Remember the biased random walk? Well there's always a reason for a bias. Bacteria can bias their walks based on the concentration gradient of a particular chemical. So even though each step is in a random direction, the length of the step is longer if the bacterium is moving towards a higher concentration than it is if the bacterium is moving towards a lower concentration.
Let's watch the biased random walk video again, this time with the concentration gradient in the background. Now you can see the reason for the bias in the walk!
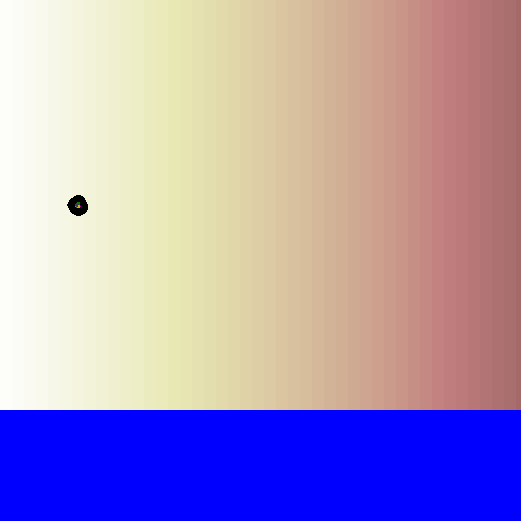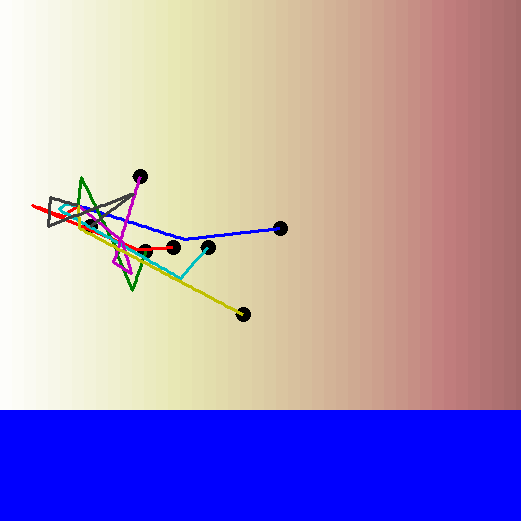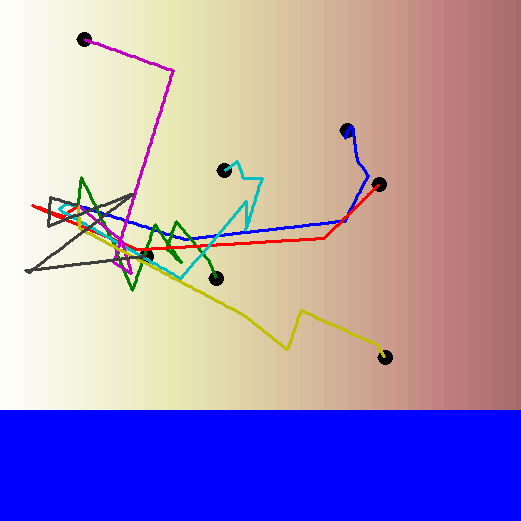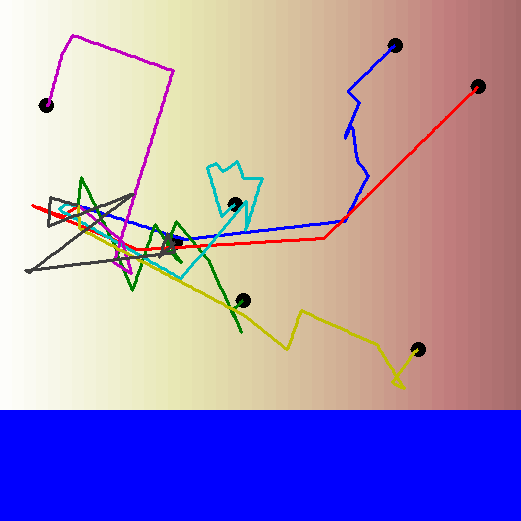
How can bacteria tell if they're moving towards higher or lower concentration?
When a bacterium is looking for a particular chemical signal, it detects this chemical as it moves along its path. If it is moving up the concentration gradient, it will start detecting the chemical's molecules more and more frequently. If it is moving down the concentration gradient, it will start detecting the chemical's molecules less and less frequently. This ultimately determines the direction and strength of the bias in its random walk.
 |