Allosteric regulation of ribonucleotide reductase
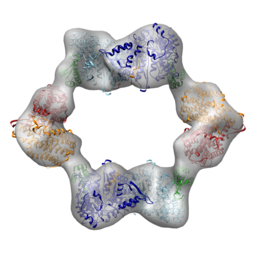 Ribonucleotide reductase (RNR) is an essential enzyme in all organisms, and as the cell’s only de novo source for deoxyribonucleotides is the target of several clinically used cancer drugs.
In many aerobic organisms (humans, yeast, and E. coli) RNR is composed of two subunits, α2 and β2.
RNR activity is coupled to poorly understood changes in oligomeric state.
We seek to define the allosteric regulation of RNR by examining its quaternary structure in the presence of nucleotide effectors and clinically used drugs.
Ribonucleotide reductase (RNR) is an essential enzyme in all organisms, and as the cell’s only de novo source for deoxyribonucleotides is the target of several clinically used cancer drugs.
In many aerobic organisms (humans, yeast, and E. coli) RNR is composed of two subunits, α2 and β2.
RNR activity is coupled to poorly understood changes in oligomeric state.
We seek to define the allosteric regulation of RNR by examining its quaternary structure in the presence of nucleotide effectors and clinically used drugs.
E. coli RNR responds to the inhibitory effector dATP by forming an α4β4 complex. In collaboration with JoAnne Stubbe’s lab at MIT and Francisco Asturias’s lab at Scripps, we recently determined the structure of the dATP-inhibited E. coli RNR. The subunits adopt an unexpected ring of alternating subunits and provided a clear rationale for allosteric inhibition. My contribution to these publications was to use electron microscopy (EM) to study RNR in the presence of dATP, establish the arrangement of subunits, and suggest a mechanism for its assembly (PNAS 2011; Structure 2012).
I've also been working with the Asturias and Stubbe labs to understand the allosteric regulation of human RNR. We recently showed that a clinically used drug, clofarabine, induces trimers of human α2 (i.e., α6) that resemble the dATP-inhibited arrangement that was recently observed in yeast RNR (ChemBiol 2012).
Fatty acid synthesis requires dramatic conformational changes
 Metazoan fatty acid synthase (FAS) is a potential target for treatment of obesity and cancer.
FAS contains all of the enzymes required for de novo fatty acid biosynthesis fused into a single ~2500 residue polypeptide.
During fatty acid synthesis the elongating acyl chain is extended and processed while covalently attached to a carrier domain that is also a member of this giant multi-enzyme protein.
FAS is a dimer with catalytic domains from each subunit arranged in a "reaction chamber" around each carrier domain. Despite an atomic-level understanding of FAS structure, it was unclear how reaction intermediates can transfer between distant catalytic domains while covalently attached to the carrier domain.
Using EM I identified a continuum of conformations (see Figure) and quantified the distribution of particles among these conformations.
The substrate loading and condensation domains (purple and blue domains) dramatically swing and swivel to access substrates.
Concomitant rearrangement of the chain processing domains in the upper portion (cyan, green, orange, and yellow domains) synchronizes acyl-chain processing (reduction and dehydration) in one reaction chamber with acyl-chain extension in the other.
These structures demonstrate that remarkable flexibility of FAS mediates the transfer of substrates between catalytic sites.
This work was published and featured on the cover of Nature Structural & Molecular Biology (NSMB 2009)
Metazoan fatty acid synthase (FAS) is a potential target for treatment of obesity and cancer.
FAS contains all of the enzymes required for de novo fatty acid biosynthesis fused into a single ~2500 residue polypeptide.
During fatty acid synthesis the elongating acyl chain is extended and processed while covalently attached to a carrier domain that is also a member of this giant multi-enzyme protein.
FAS is a dimer with catalytic domains from each subunit arranged in a "reaction chamber" around each carrier domain. Despite an atomic-level understanding of FAS structure, it was unclear how reaction intermediates can transfer between distant catalytic domains while covalently attached to the carrier domain.
Using EM I identified a continuum of conformations (see Figure) and quantified the distribution of particles among these conformations.
The substrate loading and condensation domains (purple and blue domains) dramatically swing and swivel to access substrates.
Concomitant rearrangement of the chain processing domains in the upper portion (cyan, green, orange, and yellow domains) synchronizes acyl-chain processing (reduction and dehydration) in one reaction chamber with acyl-chain extension in the other.
These structures demonstrate that remarkable flexibility of FAS mediates the transfer of substrates between catalytic sites.
This work was published and featured on the cover of Nature Structural & Molecular Biology (NSMB 2009)
Subunit architecture of transcription factor IIH (TFIIH)
 General transcription factors assemble at promoters prior to transcription initiation.
TFIIH is the only general transcription factor with enzymatic activity, containing two helicases (XPD and XPB) and a cyclin dependent kinase (TFIIK).
Mutations in these subunits result in tricothiodistrophy, Cockayne’s syndrome, and xeroderma pigmentosa.
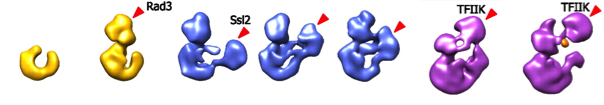
In collaboration with the Kornberg lab (Stanford) I used EM to determine structures of the complete 10-subunit yeast TFIIH and a nested set of subcomplexes, containing 5, 6, and 7 subunits (see Figure).
Consistency among the structures established the location of the 5-subunit core and additional densities could be ascribed to XPD, XPB, and the TFIIK trimer.
Based on this architecture we proposed a model of the RNA polymerase II preinitiation complex (PNAS 2012).
General transcription factors assemble at promoters prior to transcription initiation.
TFIIH is the only general transcription factor with enzymatic activity, containing two helicases (XPD and XPB) and a cyclin dependent kinase (TFIIK).
Mutations in these subunits result in tricothiodistrophy, Cockayne’s syndrome, and xeroderma pigmentosa.
In collaboration with the Kornberg lab (Stanford) I used EM to determine structures of the complete 10-subunit yeast TFIIH and a nested set of subcomplexes, containing 5, 6, and 7 subunits (see Figure).
Consistency among the structures established the location of the 5-subunit core and additional densities could be ascribed to XPD, XPB, and the TFIIK trimer.
Based on this architecture we proposed a model of the RNA polymerase II preinitiation complex (PNAS 2012).